Forecasting brain tumors
Predicting brain tumors like weather forecasters predict an upcoming storm. For patients, that means they can better prepare and make choices for how to better protect themselves.
WGN meteorologist Tom Skilling surrounds himself with mathematical data. Wind speed, barometric pressure, temperature rise and fall. Armed with that knowledge, he creates the daily and weekly forecast.
In this lab, researchers are doing the exact same thing, except they are forecasting how quickly a brain tumor will erupt and spread. It lets patients know if they should ditch the raincoat for a stronger protector from the elements.
Prof. Kristin Swanson, Northwestern Medicine Brain Researcher: “So if a patient comes in and gets some new treatment, you say ‘I predicted the disease was going to be this big at some future time point,’ and the disease was only this big, then that difference is a measure of how well the treatment worked. And that difference is actually very predictive of how well a patient does in overall survival.”
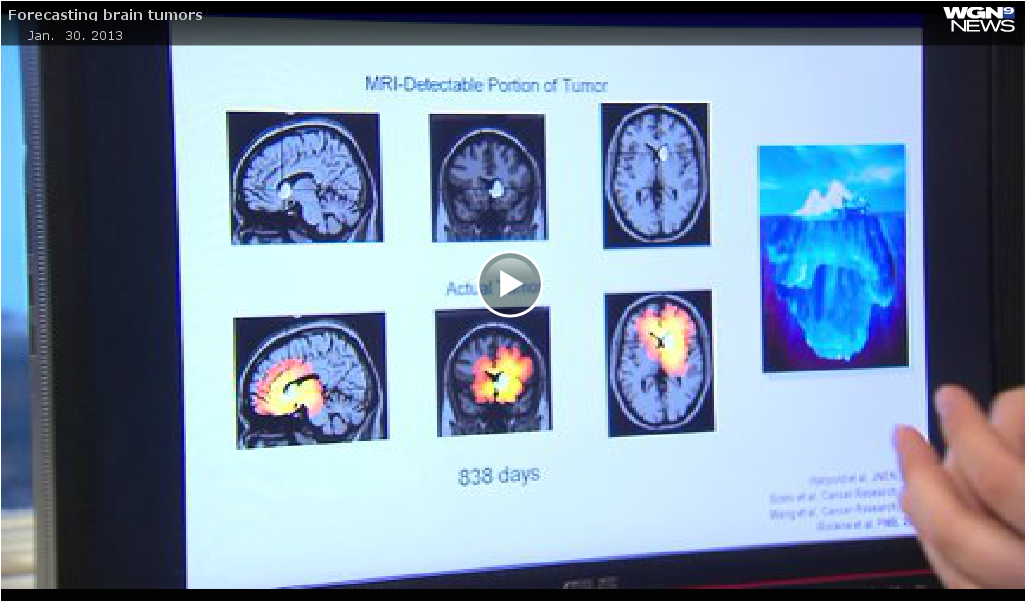
Look at this image. Think of the white spot as an iceberg, the part you can see above water. Then look at the image below. The red represents the true size — the growth and tumor spread through the brain, like the iceberg below the water.
Prof. Kristin Swanson: “It’s all about seeing what you can’t see. The imaging doesn’t tell you the whole answer so how can you infer where the disease is?”
MRI is good but limited. Instead, researchers at Northwestern Medicine are using a mathematical model comparing the rate of growth on MRI and analyzing cell density.
Prof. Kristin Swanson: “If you’ve got two pre-treatment MRI’s, you can tune the mathematical model to each patient. One of the nice things about this tool set is that you can not only predict what the disease extent is, but you can say, ‘Hey, this is what this patient looks like today, this is what I think the patient’s going to look like in six weeks, this is what I think the patient’s disease is going to look like in six months.’ And when you combine all that information together, you can generate a baseline against which to compare treatments.”
Plotting points on a graph, in this case the line represents how large and fast the tumor will grow. But then treatment is started. Now look at the red box below the line. It represents how much smaller the mass was from the original prediction. The cancer is no longer rising and growing, but the angle of progression is shrinking … the therapy is working. It tells doctors to stay the course.
Prof. Kristin Swanson: “They need to know whether the treatment that they are on is doing the right thing. So that’s one of the nice things about this tool is that it says, ‘Hey, these patients are actually getting a lot of bang for their buck, they’re getting a ton from this given therapy, where these patients over here, they may not be getting as much.’”
The ultimate goal is to accurately predict exactly when to change therapies. Currently, some patients stick with what they know without opting for a clinical trial when it may save their lives, others give up on the strongest standard therapy too soon. The researchers are hoping prediction will mean life extension.
https://wgntv.com/2013/01/30/forecasting-brain-tumors/
Donate to the
Swanson Lab